Are Muscle Cells Controlled Individually Or In Groups
| Skeletal muscle | |
|---|---|
 A superlative-downwardly view of skeletal musculus | |
| Details | |
| Synonyms | Skeletal striated muscle / Striated voluntary muscle |
| Arrangement | Muscular arrangement |
| Identifiers | |
| Latin | muscularis skeletalis |
| MeSH | D018482 |
| TH | H2.00.05.2.00002 |
| Anatomical terminology [edit on Wikidata] | |
Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular organization that are by and large attached by tendons to basic of the skeleton.[1] [ii] The muscle cells of skeletal muscles are much longer than in the other types of musculus tissue, and are often known equally musculus fibers.[3] The muscle tissue of a skeletal muscle is striated – having a striped advent due to the arrangement of the sarcomeres.
Skeletal muscles are voluntary muscles nether the command of the somatic nervous arrangement. The other types of muscle are cardiac muscle which is also striated and smoothen musculus which is non-striated; both of these types of musculus tissue are classified equally involuntary, or, under the control of the autonomic nervous organisation.[4]
A skeletal musculus contains multiple fascicles – bundles of muscle fibers. Each private fiber, and each muscle is surrounded by a type of connective tissue layer of fascia. Muscle fibers are formed from the fusion of developmental myoblasts in a process known as myogenesis resulting in long multinucleated cells. In these cells the nuclei termed myonuclei are located forth the inside of the cell membrane. Muscle fibers also take multiple mitochondria to meet free energy needs.
Musculus fibers are in turn composed of myofibrils. The myofibrils are equanimous of actin and myosin filaments called myofilaments, repeated in units called sarcomeres, which are the basic functional, contractile units of the muscle cobweb necessary for muscle contraction.[5] Muscles are predominantly powered by the oxidation of fats and carbohydrates, but anaerobic chemical reactions are also used, particularly by fast twitch fibers. These chemical reactions produce adenosine triphosphate (ATP) molecules that are used to power the movement of the myosin heads.[six]
Structure [edit]
Gross anatomy [edit]

Front view of major skeletal muscles
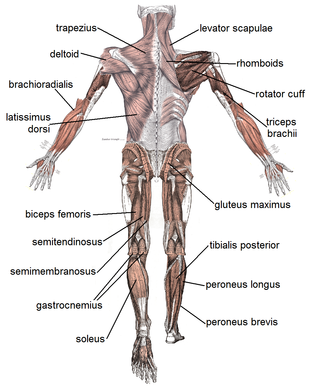
Dorsum view of major skeletal muscles
There are more than 600 skeletal muscles in the human trunk, making upwards around 40% to l% of body weight.[7] [viii] Virtually muscles occur in bilaterally-placed pairs to serve both sides of the body. Muscles are oftentimes classed as groups of muscles that piece of work together to deport out an action. In the torso at that place are several major musculus groups including the pectoral, and abdominal muscles; intrinsic and extrinsic muscles are subdivisions of muscle groups in the mitt, human foot, tongue, and extraocular muscles of the center. Muscles are too grouped into compartments including four groups in the arm, and the four groups in the leg.
Autonomously from the contractile part of a muscle consisting of its fibers, a muscle contains a non-contractile function of dumbo fibrous connective tissue that makes upwardly the tendon at each end. The tendons adhere the muscles to bones to give skeletal motility. The length of a muscle includes the tendons. Connective tissue is nowadays in all muscles every bit deep fascia. Deep fascia specialises within muscles to enclose each muscle fiber as endomysium; each musculus fascicle as perimysium, and each individual muscle as epimysium. Together these layers are chosen mysia. Deep fascia also separates the groups of muscles into muscle compartments.
Two types of sensory receptors establish in muscles are muscle spindles, and Golgi tendon organs. Muscle spindles are stretch receptors located in the muscle belly. Golgi tendon organs are proprioceptors located at the myotendinous junction that inform of a muscle's tension.
Skeletal musculus fibers [edit]

3D rendering of a skeletal muscle cobweb
Skeletal muscle cells are the private contractile cells inside a muscle, and are often termed as muscle fibers.[2] A single muscle such as the biceps in a young developed male person contains effectually 253,000 musculus fibers.[9]
Skeletal musculus fibers are the only muscle cells that are multinucleated with the nuclei often referred to as myonuclei. This occurs during myogenesis with the fusion of myoblasts each contributing a nucleus.[10] Fusion depends on muscle-specific proteins known every bit fusogens chosen myomaker and myomerger.[xi]
Many nuclei are needed past the skeletal muscle cell for the large amounts of proteins and enzymes needed to be produced for the cell's normal functioning. A single muscle fiber can comprise from hundreds to thousands of nuclei.[12] A muscle cobweb for example in the human biceps with a length of 10 cm can have equally many as 3000 nuclei.[12] Unlike in a non-muscle prison cell where the nucleus is centrally positioned, the myonucleus is elongated and located close to the sarcolemma. The myonuclei are quite uniformly arranged along the fiber with each nucleus having its own myonuclear domain where it is responsible for supporting the book of cytoplasm in that particular department of the myofiber.[11] [12]
A group of musculus stalk cells known every bit myosatellite cells, also satellite cells are found between the basement membrane and the sarcolemma of muscle fibers. These cells are normally quiescent but can be activated past practise or pathology to provide boosted myonuclei for muscle growth or repair.[13]
Attachment to tendons [edit]
Muscles attach to tendons in a complex interface region known as the musculotendinous junction also known as the myotendinous junction, an surface area specialised for the primary transmission of force.[14] At the muscle-tendon interface, force is transmitted from the sarcomeres in the muscle cells to the tendon.[5] Muscles and tendons develop in shut association, and after their joining at the myotendinous junction they establish a dynamic unit for the manual of forcefulness from muscle contraction to the skeletal arrangement.[14]
Organisation of muscle fibers [edit]

Muscle types by fiber system
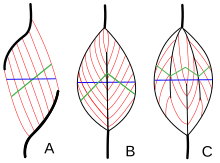
Muscle compages refers to the system of muscle fibers relative to the centrality of force generation, which runs from a musculus's origin to its insertion. The usual arrangements are types of parallel, and types of pennate muscle. In parallel muscles the fascicles run parallel to the axis of force generation, simply the fascicles can vary in their relationship to one another, and to their tendons.[15] These variations are seen in fusiform, strap, and convergent muscles.[three] A convergent muscle has a triangular or fan-shape as the fibers converge at its insertion and are fanned out broadly at the origin.[fifteen] A less common instance of a parallel muscle is a circular muscle such as the orbicularis oculi, in which the fibers are longitudinally arranged, simply create a circle from origin to insertion.[16] These different architectures, tin crusade variations in the tension that a muscle can create between its tendons.
The fibers in pennate muscles run at an bending to the centrality of force generation.[16] This pennation angle reduces the effective force of any private cobweb, every bit it is effectively pulling off-axis. However, because of this bending, more fibers can exist packed into the same muscle book, increasing the physiological cantankerous-sectional area (PCSA). This effect is known as cobweb packing, and in terms of strength generation, information technology more overcomes the efficiency-loss of the off-axis orientation. The merchandise-off comes in overall speed of muscle shortening and in the total excursion. Overall muscle shortening speed is reduced compared to cobweb shortening speed, every bit is the full distance of shortening.[16] All of these furnishings scale with pennation angle; greater angles lead to greater forcefulness due to increased fiber packing and PCSA, but with greater losses in shortening speed and excursion. Types of pennate muscle are unipennate, bipennate, and multipennate. A unipennate musculus has similarly angled fibers that are on one side of a tendon. A bipennate muscle has fibers on two sides of a tendon. Multipennate muscles take fibers that are oriented at multiple angles along the force-generating axis, and this is the most full general and most common architecture.[16]
Muscle fiber growth [edit]
Musculus fibers grow when exercised and shrink when non in use. This is due to the fact that exercise stimulates the increase in myofibrils which increment the overall size of muscle cells. Well exercised muscles can not only add together more size but can besides develop more than mitochondria, myoglobin, glycogen and a college density of capillaries. Nonetheless muscle cells cannot separate to produce new cells, and as a result there are fewer muscle cells in an adult than in a newborn.[17]
Muscle naming [edit]
There are a number of terms used in the naming of muscles including those relating to size, shape, action, location, their orientation, and their number of heads.
- Past size
- brevis means short; longus ways long; longissimus means longest; magnus means large; major means larger; maximus ways largest; minor means smaller, and minimus smallest; latissimus means widest, and vastus means huge.[18] These terms are often used later on the particular musculus such as gluteus maximus, and gluteus minimus.[19]
- Past relative shape
- deltoid means triangular; quadratus ways having 4 sides; rhomboideus ways having a rhomboid shape; teres means circular or cylindrical, and trapezius ways having a trapezoid shape;[19] serratus means saw-toothed; orbicularis means round; pectinate means comblike; piriformis means pear-shaped; platys means apartment and gracilis ways slender.[18] Examples are the pronator teres, and the pronator quadratus.
- By action
- abductor moving away from the midline; adductor moving towards the midline; depressor moving down; elevator moving upwards; flexor moving that decreases an bending; extensor moving that increment an bending or straightens; pronator moving to face downwardly; supinator moving to face up upwards;[19] internal rotator rotating towards the body; external rotator rotating away from the torso; sphincter decreases the size, and tensor gives tension to; fixator muscles serve to fix a joint in a given position past stabilizing the prime mover whilst other joints are moving.
- By number of heads
- biceps ii; triceps iii and quadriceps 4.[nineteen]
- By location
- named after the near main construction such as the temporal musculus (temporalis) most to the temporal bone.[18] Also supra- above; infra- below, and sub- under.[7]
- By fascicle orientation
- Relative to the midline, rectus means parallel to the midline; transverse ways perpendicular to the midline, and oblique ways diagonal to the midline.[18] Relative to the axis of the generation of forcefulness – types of parallel, and types of pennate muscles.
Cobweb types [edit]
Broadly in that location are two types of musculus fiber: Type I, which is slow, and Type Ii which are fast. Type Ii has 2 divisions of type IIA (oxidative), and type IIX (glycolytic), giving three primary fiber types.[xx] These fibers take relatively distinct metabolic, contractile, and motor unit properties. The tabular array below differentiates these types of properties. These types of properties—while they are partly dependent on the properties of individual fibers—tend to be relevant and measured at the level of the motor unit, rather than private fiber.[21]
| Properties | Type I fibers | Type IIA fibers | Type IIX fibers |
|---|---|---|---|
| Motor Unit Type | Slow Oxidative (And so) | Fast Oxidative/Glycolytic (FOG) | Fast Glycolytic (FG) |
| Twitch speed | Ho-hum | Fast | Fast |
| Twitch strength | Small | Medium | Large |
| Resistance to fatigue | High | High | Low |
| Glycogen content | Low | High | High |
| Capillary supply | Rich | Rich | Poor |
| Capillary density | High | Intermediate | Low |
| Myoglobin | Loftier | Loftier | Low |
| Scarlet color | Night | Nighttime | Pale |
| Mitochondrial density | Loftier | High | Low |
| Oxidative enzyme capacity | High | Intermediate-loftier | Low |
| Z-line width | Intermediate | Wide | Narrow |
| Alkali metal ATPase activeness | Low | High | High |
| Acidic ATPase activity | High | Medium-loftier | Depression |
Fiber color [edit]
Traditionally, fibers were categorized depending on their varying color, which is a reflection of myoglobin content. Type I fibers appear red due to the high levels of myoglobin. Carmine muscle fibers tend to accept more mitochondria and greater local capillary density. These fibers are more suited for endurance and are slow to fatigue because they utilize oxidative metabolism to generate ATP (adenosine triphosphate). Less oxidative Type Ii fibers are white due to relatively low myoglobin and a reliance on glycolytic enzymes.
Twitch speed [edit]
Fibers tin can also be classified on their twitch capabilities, into fast and tedious twitch. These traits largely, but not completely, overlap the classifications based on color, ATPase, or MHC.
Some authors define a fast twitch fiber equally one in which the myosin tin can divide ATP very quickly. These mainly include the ATPase blazon Two and MHC blazon II fibers. Nevertheless, fast twitch fibers also demonstrate a higher capability for electrochemical transmission of action potentials and a rapid level of calcium release and uptake by the sarcoplasmic reticulum. The fast twitch fibers rely on a well-developed, anaerobic, short term, glycolytic arrangement for energy transfer and tin can contract and develop tension at two–3 times the charge per unit of irksome twitch fibers. Fast twitch muscles are much better at generating curt bursts of forcefulness or speed than wearisome muscles, and and then fatigue more than quickly.[22]
The slow twitch fibers generate energy for ATP re-synthesis past means of a long term organisation of aerobic energy transfer. These mainly include the ATPase type I and MHC blazon I fibers. They tend to take a low activity level of ATPase, a slower speed of contraction with a less well developed glycolytic chapters.[22] Fibers that become tedious-twitch develop greater numbers of mitochondria and capillaries making them improve for prolonged piece of work.[23]
- Type distribution
Individual muscles tend to be a mixture of diverse cobweb types, just their proportions vary depending on the deportment of that musculus. For instance, in humans, the quadriceps muscles comprise ~52% type I fibers, while the soleus is ~80% type I.[24] The orbicularis oculi musculus of the heart is only ~15% type I.[24] Motor units within the muscle, nevertheless, have minimal variation between the fibers of that unit. It is this fact that makes the size chief of motor unit of measurement recruitment viable.
The total number of skeletal muscle fibers has traditionally been thought not to modify. Information technology is believed in that location are no sex or age differences in fiber distribution; however, proportions of fiber types vary considerably from muscle to muscle and person to person.[ citation needed ] Among dissimilar species there is much variation in the proportions of muscle fiber types.[25]
Sedentary men and women (besides equally young children) have 45% type Ii and 55% type I fibers.[ citation needed ] People at the higher end of any sport tend to demonstrate patterns of fiber distribution e.g. endurance athletes show a higher level of blazon I fibers. Sprint athletes, on the other hand, require large numbers of blazon IIX fibers. Middle-altitude event athletes bear witness approximately equal distribution of the two types. This is also often the case for power athletes such as throwers and jumpers. Information technology has been suggested that various types of do can induce changes in the fibers of a skeletal muscle.[26]
It is thought that if you perform endurance blazon events for a sustained period of time, some of the type IIX fibers transform into type IIA fibers. However, there is no consensus on the field of study. It may well be that the type IIX fibers show enhancements of the oxidative capacity later high intensity endurance training which brings them to a level at which they are able to perform oxidative metabolism as finer every bit slow twitch fibers of untrained subjects. This would be brought about by an increase in mitochondrial size and number and the associated related changes, non a alter in cobweb type.
Cobweb typing methods [edit]

ATPase staining of a muscle cross section. Blazon Two fibers are dark, due to the element of group i pH of the preparation. In this case, the size of the type II fibers is considerably less than the blazon I fibers due to denervation atrophy.
There are numerous methods employed for cobweb-typing, and confusion betwixt the methods is common among non-experts. Two commonly confused methods are histochemical staining for myosin ATPase activity and immunohistochemical staining for myosin heavy chain (MHC) blazon. Myosin ATPase activity is ordinarily—and correctly—referred to as just "fiber blazon", and results from the direct assaying of ATPase activity under various conditions (e.g. pH).[21] Myosin heavy concatenation staining is well-nigh accurately referred to every bit "MHC cobweb type", e.g. "MHC IIa fibers", and results from conclusion of different MHC isoforms.[21] These methods are closely related physiologically, every bit the MHC type is the primary determinant of ATPase activity. However, neither of these typing methods is directly metabolic in nature; they do non directly accost oxidative or glycolytic capacity of the cobweb.
When "blazon I" or "type 2" fibers are referred to generically, this most accurately refers to the sum of numerical fiber types (I vs. Ii) as assessed by myosin ATPase activity staining (eastward.g. "type Ii" fibers refers to type IIA + type IIAX + type IIXA ... etc.).
Below is a table showing the relationship between these two methods, limited to fiber types found in humans. Subtype capitalization is used in fiber typing vs. MHC typing, and some ATPase types actually contain multiple MHC types. Also, a subtype B or b is not expressed in humans by either method.[27] Early researchers believed humans to express a MHC IIb, which led to the ATPase classification of IIB. However, afterwards enquiry showed that the human being MHC IIb was in fact IIx,[27] indicating that the IIB is improve named IIX. IIb is expressed in other mammals, then is all the same accurately seen (along with IIB) in the literature. Non human fiber types include true IIb fibers, IIc, IId, etc.
| ATPase type | MHC heavy chain(due south) |
|---|---|
| Type I | MHC Iβ |
| Blazon IC | MHC Iβ > MHC IIa |
| Type IIC | MHC IIa > MHC Iβ |
| Type IIA | MHC IIa |
| Type IIAX | MHC IIa > MHC IIx |
| Type IIXA | MHC IIx > MHC IIa |
| Blazon IIX | MHC IIx |
Further fiber typing methods are less formally delineated, and be on more of a spectrum. They tend to be focused more than on metabolic and functional capacities (i.e., oxidative vs. glycolytic, fast vs. slow contraction time). As noted above, cobweb typing by ATPase or MHC does not directly measure or dictate these parameters. Notwithstanding, many of the diverse methods are mechanistically linked, while others are correlated in vivo.[30] [31] For instance, ATPase cobweb type is related to contraction speed, considering high ATPase activity allows faster crossbridge cycling.[21] While ATPase action is only one component of contraction speed, Type I fibers are "slow", in office, because they have depression speeds of ATPase activity in comparing to Type Two fibers. However, measuring contraction speed is not the aforementioned equally ATPase fiber typing.
Microanatomy [edit]

Construction of muscle fibre showing a sarcomere under electron microscope with schematic explanation.
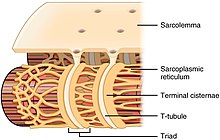
Diagram of sarcoplasmic reticulum with concluding cisternae and T-tubules.
Skeletal muscle exhibits a distinctive banding design when viewed under the microscope due to the organization of two contractile proteins myosin, and actin – that are two of the myofilaments in the myofibrils. The myosin forms the thick filaments, and actin forms the thin filaments, and are arranged in repeating units called sarcomeres. The interaction of both proteins results in muscle contraction.
The sarcomere is attached to other organelles such equally the mitochondria by intermediate filaments in the cytoskeleton. The costamere attaches the sarcomere to the sarcolemma.[5]
Every single organelle and macromolecule of a muscle fiber is arranged to ensure that information technology meets desired functions. The cell membrane is chosen the sarcolemma with the cytoplasm known as the sarcoplasm. In the sarcoplasm are the myofibrils. The myofibrils are long protein bundles about one micrometer in diameter. Pressed against the inside of the sarcolemma are the unusual flattened myonuclei. Betwixt the myofibrils are the mitochondria.
While the muscle fiber does not have smooth endoplasmic cisternae, information technology contains sarcoplasmic reticulum. The sarcoplasmic reticulum surrounds the myofibrils and holds a reserve of the calcium ions needed to cause a muscle contraction. Periodically, it has dilated end sacs known as terminal cisternae. These cantankerous the muscle fiber from one side to the other. In between ii final cisternae is a tubular infolding called a transverse tubule (T tubule). T tubules are the pathways for action potentials to signal the sarcoplasmic reticulum to release calcium, causing a musculus contraction. Together, two concluding cisternae and a transverse tubule grade a triad.[32]
Evolution [edit]

All muscles are derived from paraxial mesoderm. During embryonic development in the process of somitogenesis the paraxial mesoderm is divided forth the embryo's length to form somites, corresponding to the division of the body well-nigh apparently seen in the vertebral column.[33] Each somite has three divisions, sclerotome (which forms vertebrae), dermatome (which forms pare), and myotome (which forms muscle). The myotome is divided into ii sections, the epimere and hypomere, which class epaxial and hypaxial muscles, respectively. The simply epaxial muscles in humans are the erector spinae and small vertebral muscles, and are innervated by the dorsal rami of the spinal fretfulness. All other muscles, including those of the limbs are hypaxial, and innervated past the ventral rami of the spinal nerves.[33]
During development, myoblasts (muscle progenitor cells) either remain in the somite to form muscles associated with the vertebral column or migrate out into the body to form all other muscles. Myoblast migration is preceded by the formation of connective tissue frameworks, usually formed from the somatic lateral plate mesoderm. Myoblasts follow chemical signals to the appropriate locations, where they fuse into elongated multinucleated skeletal muscle cells.[33]
Between the 10th and the eighteenth weeks of gestation, all muscle cells have fast myosin heavy chains; two myotube types become distinguished in the developing fetus – both expressing fast chains only one expressing fast and slow bondage. Between 10 and xl per cent of the fibers express the slow myosin chain.[34]
Cobweb types are established during embryonic development and are remodelled later in the developed by neural and hormonal influences.[25] The population of satellite cells nowadays underneath the basal lamina is necessary for the postnatal development of musculus cells.[35]
Part [edit]
The chief function of muscle is contraction.[ii] Following wrinkle, skeletal muscle functions as an endocrine organ past secreting myokines – a wide range of cytokines and other peptides that human activity as signalling molecules.[36] Myokines in turn are believed to mediate the wellness benefits of do.[37] Myokines are secreted into the bloodstream after musculus contraction. Interleukin half-dozen (IL-6) is the most studied myokine, other muscle contraction-induced myokines include BDNF, FGF21, and SPARC.[38]
Musculus also functions to produce body heat. Muscle wrinkle is responsible for producing 85% of the body's heat.[39] This estrus produced is every bit a by-product of muscular activity, and is mostly wasted. Every bit a homeostatic response to extreme common cold, muscles are signaled to trigger contractions of shivering in society to generate oestrus.[twoscore]
Contraction [edit]

When a sarcomere contracts, the Z lines move closer together, and the I ring becomes smaller. The A band stays the same width. At full contraction, the thin and thick filaments overlap.
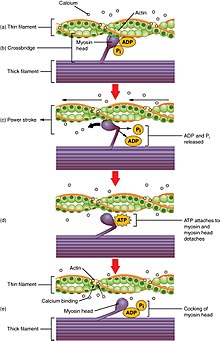
Contraction in more detail
Contraction is achieved past the musculus'southward structural unit of measurement the muscle fiber, and by its functional unit, the motor unit.[iii] Muscle fibers are excitable cells stimulated by motor neurons. The motor unit of measurement consists of a motor neuron and the many fibers that it makes contact with. A unmarried muscle is stimulated by many motor units. Muscle fibers are subject to depolarization by the neurotransmitter acetylcholine, released by the motor neurons at the neuromuscular junctions.[41]
In addition to the actin and myosin myofilaments in the myofibrils that make up the contractile sarcomeres, there are 2 other of import regulatory proteins – troponin and tropomyosin, that brand muscle contraction possible. These proteins are associated with actin and cooperate to foreclose its interaction with myosin. Once a prison cell is sufficiently stimulated, the cell'southward sarcoplasmic reticulum releases ionic calcium (Ca2+), which so interacts with the regulatory protein troponin. Calcium-bound troponin undergoes a conformational modify that leads to the movement of tropomyosin, afterwards exposing the myosin-binding sites on actin. This allows for myosin and actin ATP-dependent cantankerous-bridge cycling and shortening of the muscle.
Excitation-contraction coupling [edit]
Excitation contraction coupling is the process by which a muscular action potential in the musculus fiber causes the myofibrils to contract. This procedure relies on a direct coupling between the sarcoplasmic reticulum calcium release aqueduct RYR1 (ryanodine receptor 1), and voltage-gated L-blazon calcium channels (identified as dihydropyridine receptors, DHPRs). DHPRs are located on the sarcolemma (which includes the surface sarcolemma and the transverse tubules), while the RyRs reside across the SR membrane. The close apposition of a transverse tubule and 2 SR regions containing RyRs is described equally a triad and is predominantly where excitation–contraction coupling takes identify. Excitation–contraction coupling occurs when depolarization of skeletal muscle cell results in a muscle action potential, which spreads across the cell surface and into the musculus fiber's network of T-tubules, thereby depolarizing the inner portion of the muscle cobweb. Depolarization of the inner portions activates dihydropyridine receptors in the last cisternae, which are in close proximity to ryanodine receptors in the side by side sarcoplasmic reticulum. The activated dihydropyridine receptors physically collaborate with ryanodine receptors to actuate them via foot processes (involving conformational changes that allosterically activates the ryanodine receptors). Equally the ryanodine receptors open, Ca 2+
is released from the sarcoplasmic reticulum into the local junctional space and diffuses into the bulk cytoplasm to cause a calcium spark. Note that the sarcoplasmic reticulum has a large calcium buffering capacity partially due to a calcium-bounden protein called calsequestrin. The well-nigh synchronous activation of thousands of calcium sparks by the activity potential causes a cell-broad increase in calcium giving ascent to the upstroke of the calcium transient. The Ca 2+
released into the cytosol binds to Troponin C by the actin filaments, to permit crossbridge cycling, producing force and, in some situations, motion. The sarco/endoplasmic reticulum calcium-ATPase (SERCA) actively pumps Ca 2+
back into the sarcoplasmic reticulum. As Ca 2+
declines back to resting levels, the force declines and relaxation occurs.[42]
Muscle movement [edit]
The efferent leg of the peripheral nervous system is responsible for conveying commands to the muscles and glands, and is ultimately responsible for voluntary movement. Nerves motility muscles in response to voluntary and autonomic (involuntary) signals from the brain. Deep muscles, superficial muscles, muscles of the face and internal muscles all correspond with dedicated regions in the chief motor cortex of the encephalon, directly anterior to the cardinal sulcus that divides the frontal and parietal lobes.
In addition, muscles react to reflexive nerve stimuli that do not e'er ship signals all the manner to the brain. In this case, the bespeak from the afferent fiber does non attain the brain, but produces the reflexive movement by direct connections with the efferent nerves in the spine. However, the bulk of muscle action is volitional, and the effect of complex interactions between various areas of the brain.
Nerves that control skeletal muscles in mammals stand for with neuron groups along the primary motor cortex of the encephalon'southward cerebral cortex. Commands are routed through the basal ganglia and are modified past input from the cerebellum before being relayed through the pyramidal tract to the spinal cord and from in that location to the motor end plate at the muscles. Forth the way, feedback, such equally that of the extrapyramidal system contribute signals to influence muscle tone and response.
Deeper muscles such as those involved in posture often are controlled from nuclei in the brain stalk and basal ganglia.
Proprioception [edit]
In skeletal muscles, muscle spindles convey information nigh the degree of musculus length and stretch to the central nervous system to assistance in maintaining posture and joint position. The sense of where our bodies are in infinite is called proprioception, the perception of body awareness, the "unconscious" awareness of where the various regions of the body are located at whatever one fourth dimension. Several areas in the brain coordinate movement and position with the feedback information gained from proprioception. The cerebellum and red nucleus in detail continuously sample position confronting motion and make pocket-size corrections to assure polish motion.[ citation needed ]
Energy consumption [edit]
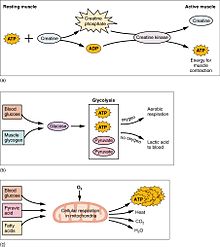
(a) Some ATP is stored in a resting muscle. As contraction starts, it is used up in seconds. More ATP is generated from creatine phosphate for about 15 seconds. (b) Each glucose molecule produces two ATP and two molecules of pyruvic acid, which can be used in aerobic respiration or converted to lactic acrid. If oxygen is non available, pyruvic acid is converted to lactic acid, which may contribute to muscle fatigue. This occurs during strenuous exercise when high amounts of energy are needed but oxygen cannot be sufficiently delivered to muscle. (c) Aerobic respiration is the breakdown of glucose in the presence of oxygen (O2) to produce carbon dioxide, h2o, and ATP. Approximately 95 percent of the ATP required for resting or moderately agile muscles is provided by aerobic respiration, which takes place in mitochondria.
Muscular action accounts for much of the torso's energy consumption. All muscle cells produce adenosine triphosphate (ATP) molecules which are used to power the movement of the myosin heads. Muscles have a short-term store of energy in the grade of creatine phosphate which is generated from ATP and tin regenerate ATP when needed with creatine kinase. Muscles also keep a storage class of glucose in the form of glycogen. Glycogen tin can be speedily converted to glucose when energy is required for sustained, powerful contractions. Within the voluntary skeletal muscles, the glucose molecule can be metabolized anaerobically in a procedure called glycolysis which produces ii ATP and ii lactic acrid molecules in the process (note that in aerobic conditions, lactate is not formed; instead pyruvate is formed and transmitted through the citric acid cycle). Musculus cells also contain globules of fatty, which are used for energy during aerobic exercise. The aerobic energy systems accept longer to produce the ATP and attain superlative efficiency, and requires many more biochemical steps, but produces significantly more ATP than anaerobic glycolysis. Cardiac musculus on the other hand, can readily consume any of the three macronutrients (protein, glucose and fatty) aerobically without a 'warm up' flow and e'er extracts the maximum ATP yield from any molecule involved. The center, liver and red blood cells volition also consume lactic acid produced and excreted by skeletal muscles during practice.
Skeletal musculus uses more calories than other organs.[43] At rest information technology consumes 54.4 kJ/kg (xiii.0 kcal/kg) per day. This is larger than adipose tissue (fat) at 18.eight kJ/kg (four.v kcal/kg), and bone at nine.half dozen kJ/kg (ii.3 kcal/kg).[44]
Efficiency [edit]
The efficiency of man muscle has been measured (in the context of rowing and cycling) at eighteen% to 26%. The efficiency is defined as the ratio of mechanical work output to the full metabolic cost, equally can exist calculated from oxygen consumption. This low efficiency is the result of about 40% efficiency of generating ATP from food energy, losses in converting energy from ATP into mechanical piece of work inside the muscle, and mechanical losses inside the body. The latter 2 losses are dependent on the blazon of exercise and the type of muscle fibers existence used (fast-twitch or slow-twitch). For an overall efficiency of 20 percent, one watt of mechanical power is equivalent to iv.3 kcal per hour. For example, one manufacturer of rowing equipment calibrates its rowing ergometer to count burned calories equally equal to iv times the bodily mechanical work, plus 300 kcal per hour, this amounts to about twenty percent efficiency at 250 watts of mechanical output. The mechanical energy output of a cyclic contraction can depend upon many factors, including activation timing, muscle strain trajectory, and rates of force rise & decay. These can be synthesized experimentally using work loop assay.
Muscle force [edit]
Muscle strength is a result of three overlapping factors: physiological forcefulness (muscle size, cross sectional area, available crossbridging, responses to training), neurological strength (how strong or weak is the signal that tells the muscle to contract), and mechanical strength (muscle'due south force angle on the lever, moment arm length, joint capabilities).[ citation needed ]
| Class 0 | No wrinkle |
| Grade i | Trace of wrinkle, just no move at the articulation |
| Grade two | Motility at the articulation with gravity eliminated |
| Grade iii | Movement against gravity, but not against added resistance |
| Grade 4 | Move against external resistance, merely less than normal |
| Grade 5 | Normal strength |
Vertebrate musculus typically produces approximately 25–33 N (5.6–vii.4 lbf) of force per square centimeter of muscle cantankerous-exclusive area when isometric and at optimal length.[45] Some invertebrate muscles, such every bit in crab claws, have much longer sarcomeres than vertebrates, resulting in many more sites for actin and myosin to demark and thus much greater forcefulness per square centimeter at the cost of much slower speed. The force generated by a contraction can be measured non-invasively using either mechanomyography or phonomyography, be measured in vivo using tendon strain (if a prominent tendon is present), or be measured straight using more invasive methods.
The forcefulness of whatsoever given muscle, in terms of force exerted on the skeleton, depends upon length, shortening speed, cantankerous exclusive surface area, pennation, sarcomere length, myosin isoforms, and neural activation of motor units. Significant reductions in muscle strength can indicate underlying pathology, with the chart at correct used equally a guide.
The maximum holding time for a contracted muscle depends on its supply of energy and is stated by Rohmert'due south police to exponentially disuse from the showtime of exertion.
The "strongest" human muscle [edit]
Since three factors bear on muscular strength simultaneously and muscles never work individually, it is misleading to compare forcefulness in individual muscles, and state that one is the "strongest". But beneath are several muscles whose strength is noteworthy for dissimilar reasons.
- In ordinary parlance, muscular "strength" unremarkably refers to the power to exert a strength on an external object—for case, lifting a weight. By this definition, the masseter or jaw musculus is the strongest. The 1992 Guinness Book of Records records the achievement of a bite forcefulness of 4,337 N (975 lbf) for 2 seconds. What distinguishes the masseter is not anything special most the muscle itself, merely its reward in working against a much shorter lever arm than other muscles.
- If "strength" refers to the force exerted by the musculus itself, e.g., on the place where it inserts into a bone, then the strongest muscles are those with the largest cross-sectional area. This is because the tension exerted by an individual skeletal musculus cobweb does non vary much. Each fiber can exert a force on the order of 0.iii micronewton. By this definition, the strongest muscle of the body is usually said to be the quadriceps femoris or the gluteus maximus.
- Because muscle strength is determined by cantankerous-exclusive expanse, a shorter muscle volition exist stronger "pound for pound" (i.due east., by weight) than a longer musculus of the same cross-sectional area. The myometrial layer of the uterus may be the strongest muscle by weight in the female man body. At the time when an babe is delivered, the entire human uterus weighs most 1.1 kg (forty oz). During childbirth, the uterus exerts 100 to 400 N (25 to 100 lbf) of downward forcefulness with each contraction.
- The external muscles of the eye are clearly large and stiff in relation to the modest size and weight of the eyeball. It is frequently said that they are "the strongest muscles for the task they have to do" and are sometimes claimed to exist "100 times stronger than they need to be." All the same, eye movements (peculiarly saccades used on facial scanning and reading) do require high speed movements, and eye muscles are exercised nightly during rapid centre movement sleep.
- The statement that "the natural language is the strongest musculus in the trunk" appears ofttimes in lists of surprising facts, but information technology is difficult to notice whatsoever definition of "strength" that would make this statement truthful. Note that the tongue consists of viii muscles, not one.
Strength generation [edit]
Muscle force is proportional to physiological cross-sectional area (PCSA), and muscle velocity is proportional to muscle cobweb length.[46] The torque effectually a joint, however, is determined by a number of biomechanical parameters, including the altitude between muscle insertions and pivot points, muscle size and architectural gear ratio. Muscles are usually arranged in opposition then that when one group of muscles contracts, another group relaxes or lengthens.[47] Antagonism in the transmission of nerve impulses to the muscles means that it is impossible to fully stimulate the contraction of ii antagonistic muscles at whatsoever one time. During ballistic motions such as throwing, the antagonist muscles deed to 'restriction' the agonist muscles throughout the contraction, particularly at the terminate of the motion. In the example of throwing, the chest and front of the shoulder (anterior deltoid) contract to pull the arm forward, while the muscles in the back and rear of the shoulder (posterior deltoid) also contract and undergo eccentric contraction to slow the movement downwardly to avert injury. Part of the training procedure is learning to relax the antagonist muscles to increase the forcefulness input of the chest and anterior shoulder.
Contracting muscles produce vibration and audio.[48] Slow twitch fibers produce ten to xxx contractions per second (10 to 30 Hz). Fast twitch fibers produce thirty to lxx contractions per second (30 to 70 Hz).[49] The vibration can be witnessed and felt by highly tensing ane'due south muscles, equally when making a firm fist. The audio can be heard by pressing a highly tensed muscle against the ear, again a firm fist is a practiced instance. The audio is commonly described equally a rumbling sound. Some individuals tin can voluntarily produce this rumbling sound by contracting the tensor tympani musculus of the center ear. The rumbling sound can also be heard when the neck or jaw muscles are highly tensed.[ commendation needed ]
Signal transduction pathways [edit]
Skeletal muscle fiber-type phenotype in adult animals is regulated by several independent signaling pathways. These include pathways involved with the Ras/mitogen-activated poly peptide kinase (MAPK) pathway, calcineurin, calcium/calmodulin-dependent poly peptide kinase Iv, and the peroxisome proliferator γ coactivator ane (PGC-1). The Ras/MAPK signaling pathway links the motor neurons and signaling systems, coupling excitation and transcription regulation to promote the nerve-dependent induction of the deadening program in regenerating muscle. Calcineurin, a Catwo+/calmodulin-activated phosphatase implicated in nerve activity-dependent fiber-type specification in skeletal muscle, directly controls the phosphorylation country of the transcription factor NFAT, assuasive for its translocation to the nucleus and leading to the activation of slow-blazon muscle proteins in cooperation with myocyte enhancer factor 2 (MEF2) proteins and other regulatory proteins. Ca2+/calmodulin-dependent protein kinase activeness is as well upregulated by tiresome motor neuron activity, possibly because it amplifies the slow-type calcineurin-generated responses by promoting MEF2 transactivator functions and enhancing oxidative chapters through stimulation of mitochondrial biogenesis.
Contraction-induced changes in intracellular calcium or reactive oxygen species provide signals to diverse pathways that include the MAPKs, calcineurin and calcium/calmodulin-dependent protein kinase Four to actuate transcription factors that regulate gene expression and enzyme activeness in skeletal muscle.

Do-induced signaling pathways in skeletal muscle that decide specialized characteristics of ho-hum- and fast-twitch muscle fibers
PGC1-α (PPARGC1A), a transcriptional coactivator of nuclear receptors important to the regulation of a number of mitochondrial genes involved in oxidative metabolism, straight interacts with MEF2 to synergistically activate selective slow twitch (ST) musculus genes and also serves as a target for calcineurin signaling. A peroxisome proliferator-activated receptor δ (PPARδ)-mediated transcriptional pathway is involved in the regulation of the skeletal muscle fiber phenotype. Mice that harbor an activated form of PPARδ brandish an "endurance" phenotype, with a coordinated increase in oxidative enzymes and mitochondrial biogenesis and an increased proportion of ST fibers. Thus—through functional genomics—calcineurin, calmodulin-dependent kinase, PGC-1α, and activated PPARδ form the footing of a signaling network that controls skeletal muscle fiber-type transformation and metabolic profiles that protect against insulin resistance and obesity.
The transition from aerobic to anaerobic metabolism during intense work requires that several systems are quickly activated to ensure a constant supply of ATP for the working muscles. These include a switch from fat-based to sugar-based fuels, a redistribution of blood flow from nonworking to exercising muscles, and the removal of several of the by-products of anaerobic metabolism, such as carbon dioxide and lactic acid. Some of these responses are governed by transcriptional control of the fast twitch (FT) glycolytic phenotype. For example, skeletal muscle reprogramming from an ST glycolytic phenotype to an FT glycolytic phenotype involves the Six1/Eya1 complex, equanimous of members of the Six poly peptide family. Moreover, the hypoxia-inducible factor 1-α (HIF1A) has been identified as a master regulator for the expression of genes involved in essential hypoxic responses that maintain ATP levels in cells. Ablation of HIF-1α in skeletal musculus was associated with an increase in the activity of rate-limiting enzymes of the mitochondria, indicating that the citric acid cycle and increased fatty acrid oxidation may be compensating for decreased flow through the glycolytic pathway in these animals. Yet, hypoxia-mediated HIF-1α responses are likewise linked to the regulation of mitochondrial dysfunction through the formation of excessive reactive oxygen species in mitochondria.
Other pathways likewise influence adult muscle grapheme. For example, physical force inside a muscle cobweb may release the transcription factor serum response factor from the structural protein titin, leading to altered muscle growth.
Exercise [edit]

Jogging is one grade of aerobic do.
Physical exercise is oft recommended as a ways of improving motor skills, fitness, muscle and bone forcefulness, and articulation function. Exercise has several effects upon muscles, connective tissue, bone, and the nerves that stimulate the muscles. One such result is muscle hypertrophy, an increase in size of muscle due to an increase in the number of muscle fibers or cross-sectional area of myofibrils.[50] Muscle changes depend on the blazon of exercise used.
Generally, there are 2 types of exercise regimes, aerobic and anaerobic. Aerobic exercise (e.chiliad. marathons) involves activities of depression intensity but long duration, during which the muscles used are beneath their maximal contraction strength. Aerobic activities rely on aerobic respiration (i.east. citric acid cycle and electron transport chain) for metabolic energy by consuming fat, protein, carbohydrates, and oxygen. Muscles involved in aerobic exercises contain a college percentage of Type I (or irksome-twitch) muscle fibers, which primarily contain mitochondrial and oxidation enzymes associated with aerobic respiration.[51] [52] On the contrary, anaerobic exercise is associated with exercise or short elapsing but loftier intensity (e.k. sprinting and weight lifting). The anaerobic activities predominately utilise Blazon 2, fast-twitch, muscle fibers.[53] Type II muscle fibers rely on glucogenesis for energy during anaerobic practise.[54] During anaerobic exercise, type 2 fibers consume footling oxygen, protein and fat, produces big amounts of lactic acid and are fatigable. Many exercises are partially aerobic and anaerobic; for case, soccer and rock climbing.
The presence of lactic acid has an inhibitory effect on ATP generation within the muscle. It can even cease ATP production if the intracellular concentration becomes too high. However, endurance training mitigates the buildup of lactic acid through increased capillarization and myoglobin.[55] This increases the ability to remove waste products, similar lactic acrid, out of the muscles in order to not impair muscle function. One time moved out of muscles, lactic acid tin be used by other muscles or body tissues as a source of energy, or transported to the liver where it is converted back to pyruvate. In addition to increasing the level of lactic acrid, strenuous exercise results in the loss of potassium ions in musculus. This may facilitate the recovery of muscle function by protecting confronting fatigue.[56]
Delayed onset muscle soreness is hurting or discomfort that may exist felt i to three days later exercising and mostly subsides ii to three days subsequently. One time idea to be caused past lactic acid build-up, a more recent theory is that it is caused by tiny tears in the muscle fibers caused by eccentric contraction, or unaccustomed preparation levels. Since lactic acrid disperses adequately apace, information technology could non explain hurting experienced days after exercise.[57]
Clinical significance [edit]
Muscle disease [edit]
Diseases of skeletal muscle are termed myopathies, while diseases of nerves are chosen neuropathies. Both can affect muscle role or crusade muscle pain, and autumn under the umbrella of neuromuscular disease. The cause of many myopathies is attributed to mutations in the various associated muscle proteins.[5] [58] Some inflammatory myopathies include polymyositis and inclusion body myositis

Neuromuscular diseases affect the muscles and their nervous command. In full general, problems with nervous control can cause spasticity or paralysis, depending on the location and nature of the trouble. A number of motion disorders are caused past neurological disorders such every bit Parkinson'south disease and Huntington's illness where there is cardinal nervous organization dysfunction.[59]
Symptoms of muscle diseases may include weakness, spasticity, myoclonus and myalgia. Diagnostic procedures that may reveal muscular disorders include testing creatine kinase levels in the claret and electromyography (measuring electrical activeness in muscles). In some cases, musculus biopsy may be done to identify a myopathy, every bit well as genetic testing to identify DNA abnormalities associated with specific myopathies and dystrophies.
A non-invasive elastography technique that measures muscle noise is undergoing experimentation to provide a way of monitoring neuromuscular disease. The sound produced past a muscle comes from the shortening of actomyosin filaments forth the axis of the muscle. During contraction, the muscle shortens forth its length and expands across its width, producing vibrations at the surface.[60]
Hypertrophy [edit]
Independent of strength and performance measures, muscles can be induced to grow larger past a number of factors, including hormone signaling, developmental factors, strength preparation, and disease. Reverse to pop belief, the number of muscle fibres cannot be increased through exercise. Instead, muscles grow larger through a combination of muscle prison cell growth as new protein filaments are added along with additional mass provided by undifferentiated satellite cells alongside the existing muscle cells.[61]
Biological factors such as age and hormone levels can affect musculus hypertrophy. During puberty in males, hypertrophy occurs at an accelerated rate as the levels of growth-stimulating hormones produced by the trunk increase. Natural hypertrophy normally stops at full growth in the late teens. As testosterone is one of the torso's major growth hormones, on average, men find hypertrophy much easier to reach than women. Taking additional testosterone or other anabolic steroids will increase muscular hypertrophy.
Muscular, spinal and neural factors all affect muscle building. Sometimes a person may notice an increase in force in a given musculus even though just its opposite has been subject to exercise, such as when a bodybuilder finds her left biceps stronger after completing a regimen focusing simply on the right biceps. This phenomenon is called cross instruction.[ citation needed ]
Atrophy [edit]

Pw exhibiting musculus loss as a result of malnutrition.
Every mean solar day betwixt one and two percent of muscle is broken down and rebuilt. Inactivity, malnutrition, affliction, and aging can increase the breakdown leading to muscle cloudburst or sarcopenia. Sarcopenia is commonly an age-related process that can cause frailty and its consequences.[62] A decrease in muscle mass may be accompanied past a smaller number and size of the muscle cells as well every bit lower protein content.[63]
Human spaceflight, involving prolonged periods of immobilization and weightlessness is known to upshot in muscle weakening and atrophy resulting in a loss of equally much every bit thirty% of mass in some muscles.[64] [65] Such consequences are also noted in some mammals post-obit hibernation.[66]
Many diseases and weather condition including cancer, AIDS, and heart failure can crusade muscle loss known every bit cachexia.[67]
Research [edit]
Myopathies accept been modeled with cell civilisation systems of muscle from healthy or diseased tissue biopsies. Some other source of skeletal muscle and progenitors is provided by the directed differentiation of pluripotent stem cells.[68] Research on skeletal musculus backdrop uses many techniques. Electrical musculus stimulation is used to determine force and contraction speed at different frequencies related to cobweb-blazon limerick and mix within an individual muscle grouping. In vitro musculus testing is used for more complete label of muscle backdrop.
The electrical activeness associated with muscle wrinkle is measured via electromyography (EMG). Skeletal muscle has two physiological responses: relaxation and wrinkle. The mechanisms for which these responses occur generate electrical activity measured by EMG. Specifically, EMG tin can mensurate the activity potential of a skeletal muscle, which occurs from the hyperpolarization of the motor axons from nerve impulses sent to the muscle. EMG is used in enquiry for determining if the skeletal muscle of involvement is being activated, the amount of forcefulness generated, and an indicator of muscle fatigue.[69] The two types of EMG are intra-muscular EMG and the most common, surface EMG. The EMG signals are much greater when a skeletal muscle is contracting verses relaxing. However, for smaller and deeper skeletal muscles the EMG signals are reduced and therefore are viewed every bit a less valued technique for measuring the activation.[70] In research using EMG, a maximal voluntary contraction (MVC) is commonly performed on the skeletal muscle of involvement, to have reference data for the residuum of the EMG recordings during the master experimental testing for that same skeletal muscle.[71]
Research into the development of artificial muscles includes the utilise of electroactive polymers.
Run into also [edit]
- Facioscapulohumeral muscular dystrophy
- Hill's muscle model
- In vitro musculus testing
- Musculoskeletal injury
- Musculus relaxant
- Microtrauma
- Muscle retention
- Myomere
- Myotomy
- Preflexes
References [edit]
- ^ Betts, J. Gordon; Immature, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H.; Korol, Oksana; Johnson, Jody E.; Womble, Mark; Desaix, Peter (6 March 2013). "Interactions of Skeletal Muscles, Their Fascicle Organisation, and Their Lever Systems". Interactions of skeletal muscles. OpenStax. Retrieved 24 May 2021.
- ^ a b c "Structure of Skeletal Muscle | SEER Training". training.seer.cancer.gov.
- ^ a b c Moore, Keith L. (2018). Clinically oriented anatomy (Eighth ed.). Philadelphia: Wolters Kluwer. pp. 30–33. ISBN9781496347213.
- ^ Birbrair, Alexander; Zhang, Tan; Wang, Zhong-Min; Messi, Maria Laura; Enikolopov, Grigori N.; Mintz, Akiva; Delbono, Osvaldo (21 March 2013). "Role of Pericytes in Skeletal Muscle Regeneration and Fatty Accumulation". Stem Cells and Evolution. 22 (16): 2298–2314. doi:x.1089/scd.2012.0647. ISSN 1547-3287. PMC3730538. PMID 23517218.
- ^ a b c d Henderson, CA; Gomez, CG; Novak, SM; Mi-Mi, L; Gregorio, CC (18 June 2017). "Overview of the Musculus Cytoskeleton". Comprehensive Physiology. 7 (3): 891–944. doi:10.1002/cphy.c160033. PMC5890934. PMID 28640448.
- ^ Brainard, Jean; Gray-Wilson, Niamh; Harwood, Jessica; Karasov, Corliss; Kraus, Dors; Willan, Jane (2011). CK-12 Life Science Honors for Centre Schoolhouse. CK-12 Foundation. p. 451. Retrieved 18 April 2015.
- ^ a b "Musculus Groups | SEER Preparation". grooming.seer.cancer.gov . Retrieved 17 May 2021.
- ^ "What is the strongest muscle in the human torso?". Library of Congress . Retrieved 17 May 2021.
- ^ Klein, CS; Marsh, GD; Petrella, RJ; Rice, CL (July 2003). "Muscle cobweb number in the biceps brachii musculus of immature and onetime men". Musculus & Nerve. 28 (1): 62–eight. doi:10.1002/mus.10386. PMID 12811774. S2CID 20508198.
- ^ Cho, CH; Lee, KJ; Lee, EH (August 2018). "With the greatest intendance, stromal interaction molecule (STIM) proteins verify what skeletal muscle is doing". BMB Reports. 51 (viii): 378–387. doi:10.5483/bmbrep.2018.51.8.128. PMC6130827. PMID 29898810.
- ^ a b Prasad, V; Millay, DP (eight May 2021). "Skeletal musculus fibers count on nuclear numbers for growth". Seminars in Cell & Developmental Biology. 119: 3–x. doi:10.1016/j.semcdb.2021.04.015. PMC 9070318. PMID 33972174. S2CID 234362466.
- ^ a b c Snijders, T; Aussieker, T; Holwerda, A; Parise, Yard; van Loon, LJC; Verdijk, LB (July 2020). "The concept of skeletal muscle retentiveness: Evidence from animal and human being studies". Acta Physiologica. 229 (3): e13465. doi:10.1111/apha.13465. PMC7317456. PMID 32175681.
- ^ Quarta, M; Cromie, One thousand; Chacon, R (xx June 2017). "Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss". Nature Communications. 8: 15613. Bibcode:2017NatCo...815613Q. doi:x.1038/ncomms15613. PMC5481841. PMID 28631758.
- ^ a b Charvet, B; Ruggiero, F; Le Guellec, D (April 2012). "The development of the myotendinous junction. A review". Muscles, Ligaments and Tendons Periodical. two (2): 53–63. PMC3666507. PMID 23738275.
- ^ a b Martini, Frederic H.; Timmons, Michael J.; Tallitsch, Robert B. (2008). Human Anatomy (6 ed.). Benjamin Cummings. pp. 251–252. ISBN978-0-321-50042-7.
- ^ a b c d Lieber, Richard L. (2002) Skeletal muscle structure, function, and plasticity. Wolters Kluwer Wellness.
- ^ Ziser, Stephen. "&Musculus Cell Beefcake & Function" (PDF). world wide web.austincc.edu. Archived (PDF) from the original on 23 September 2015. Retrieved 12 February 2015.
- ^ a b c d Tortora, Gerard J. (2012). Principles of anatomy & physiology (13th ed.). Hoboken, NJ: Wiley. p. 372. ISBN9780470646083.
- ^ a b c d Saladin, Kenneth Due south. (2011). Human anatomy (3rd ed.). New York: McGraw-Hill. p. 265. ISBN9780071222075.
- ^ Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H.; Korol, Oksana; Johnson, Jody E.; Womble, Marker; Desaix, Peter (half-dozen March 2013). Types of muscle fibers. OpenStax. Retrieved 17 June 2021.
- ^ a b c d e f MacIntosh, Brian R.; Gardiner, Phillip F.; McComas, Alan J. (2006). Skeletal Muscle: Form and Role. Human Kinetics. ISBN978-0-7360-4517-ix.
- ^ a b "Musculus cobweb type". About.com. Sports Medicine. Archived from the original on 21 November 2007. Retrieved 27 November 2007.
- ^ MacIntosh, Brian R. (2006). Skeletal muscle : form and office (2nd ed.). Champaign, IL: Human being Kinetics. p. 274. ISBN978-0-7360-4517-9.
- ^ a b Johnson, M.A.; Polgar, J.; Weightman, D.; Appleton, D. (1973). "Data on the distribution of fibre types in thirty-six human muscles. An autopsy study". Journal of the Neurological Sciences. 18 (1): 111–129. doi:10.1016/0022-510x(73)90023-3. PMID 4120482.
- ^ a b Schiaffino, Due south; Reggiani, C (October 2011). "Fiber types in mammalian skeletal muscles". Physiological Reviews. 91 (4): 1447–531. doi:ten.1152/physrev.00031.2010. PMID 22013216.
- ^ Michael Yessis (2006). Build A Amend Athlete. Ultimate Athlete Concepts. ISBN978-1-930546-78-3.
- ^ a b Smerdu, V.; Karsch-Mizrachi, I; Campione, Chiliad; Leinwand, L; Schiaffino, Southward (Dec 1994). "Type IIx myosin heavy chain transcripts are expressed in blazon IIb fibers of human skeletal muscle". The American Journal of Physiology. 267 (6 Pt 1): C1723–8. doi:x.1152/ajpcell.1994.267.six.C1723. PMID 7545970.
- ^ Pette, D; Staron, RS (xv September 2000). "Myosin isoforms, muscle fiber types, and transitions". Microscopy Research and Technique. 50 (6): 500–9. doi:10.1002/1097-0029(20000915)fifty:6<500::AID-JEMT7>three.0.CO;2-7. PMID 10998639. S2CID 7820419.
- ^ Staron, Robert S.; Johnson, Peter (Nov 1993). "Myosin polymorphism and differential expression in adult human being skeletal muscle". Comparative Biochemistry and Physiology B. 106 (iii): 463–475. doi:10.1016/0305-0491(93)90120-T. PMID 8281747.
- ^ Buchthal, F.; Schmalbruch, H. (Baronial 1970). "Contraction times and fibre types in intact human muscle". Acta Physiologica Scandinavica. 79 (4): 435–452. doi:10.1111/j.1748-1716.1970.tb04744.x. PMID 5472111.
- ^ Garnett, R.A.; O'Donovan, Grand.J.; Stephens, J.A.; Taylor, A. (February 1979). "Motor unit organization of human medial gastrocnemius". The Journal of Physiology. 287 (1): 33–43. doi:10.1113/jphysiol.1979.sp012643. PMC1281479. PMID 430414. [ permanent expressionless link ]
- ^ Saladin, Kenneth S. (2010). Beefcake and Physiology (third ed.). New York: Watnick. pp. 405–406. ISBN9780072943689.
- ^ a b c Sweeney, Lauren (1997). Basic Concepts in Embryology: A Pupil'due south Survival Guide (1st Paperback ed.). McGraw-Hill Professional.
- ^ MacIntosh, Brian R. (2006). Skeletal muscle : form and function (2d ed.). Champaign, IL: Human Kinetics. pp. 63–64. ISBN978-0-7360-4517-9.
- ^ Murach, KA; Fry, CS; Kirby, TJ; Jackson, JR; Lee, JD; White, SH; Dupont-Versteegden, EE; McCarthy, JJ; Peterson, CA (1 January 2018). "Starring or Supporting Office? Satellite Cells and Skeletal Muscle Fiber Size Regulation". Physiology. 33 (one): 26–38. doi:x.1152/physiol.00019.2017. PMC5866409. PMID 29212890.
- ^ Grube, L; Dellen, R; Kruse, F (2 February 2018). "Mining the Secretome of C2C12 Muscle Cells: Data Dependent Experimental Approach To Analyze Poly peptide Secretion Using Label-Free Quantification and Peptide Based Analysis". Journal of Proteome Research. 17 (2): 879–890. doi:10.1021/acs.jproteome.7b00684. PMID 29322779.
- ^ Pedersen, B. K. (2013). "Musculus as a Secretory Organ". Comprehensive Physiology. Comprehensive Physiology. Vol. 3. pp. 1337–62. doi:10.1002/cphy.c120033. ISBN9780470650714. PMID 23897689.
- ^ Lee, JH; Jun, HS (2019). "Role of Myokines in Regulating Skeletal Muscle Mass and Function". Frontiers in Physiology. x: 42. doi:10.3389/fphys.2019.00042. PMC6363662. PMID 30761018.
- ^ "Introduction to the Muscular System | SEER Training". preparation.seer.cancer.gov.
- ^ "1.v Homeostasis - Beefcake and Physiology | OpenStax". openstax.org . Retrieved 25 June 2021.
- ^ Costanzo, Linda S. (2002). Physiology (2nd ed.). Philadelphia: Saunders. p. 23. ISBN0-7216-9549-iii.
- ^ Calderón, Juan C.; Bolaños, Pura; Caputo, Carlo (24 Jan 2014). "The excitation–contraction coupling mechanism in skeletal muscle". Biophysical Reviews. half-dozen (1): 133–160. doi:10.1007/s12551-013-0135-ten. ISSN 1867-2450. PMC5425715. PMID 28509964.
- ^ Heydemann, A (20 June 2018). "Skeletal Musculus Metabolism in Duchenne and Becker Muscular Dystrophy-Implications for Therapies". Nutrients. 10 (6): 796. doi:10.3390/nu10060796. PMC6024668. PMID 29925809.
- ^ Heymsfield, SB; Gallagher, D; Kotler, DP; Wang, Z; Allison, DB; Heshka, South (2002). "Body-size dependence of resting energy expenditure can exist attributed to nonenergetic homogeneity of fatty-gratis mass". American Journal of Physiology. Endocrinology and Metabolism. 282 (1): E132–E138. doi:10.1152/ajpendo.2002.282.i.e132. PMID 11739093.
- ^ McGinnis, Peter M. (2013). Biomechanics of Sport and Practice (3rd ed.). Champaign, IL: Human Kinetics. ISBN978-0-7360-7966-2.
- ^ Quoted from National Skeletal Muscle Research Center; UCSD, Musculus Physiology Domicile Page – Skeletal Muscle Architecture, Effect of Muscle Architecture on Muscle Function
- ^ "9.6 Forces and Torques in Muscles and Joints - College Physics | OpenStax". openstax.org . Retrieved 15 May 2021.
- ^ Barry, D. T. (1992). "Vibrations and sounds from evoked muscle twitches". Electromyogr Clin Neurophysiol. 32 (ane–2): 35–xl. PMID 1541245.
- ^ [ane], Tiptop Performance – Endurance training: understanding your slow twitch muscle fibers will boost performance
- ^ Gonyea WJ, Sale DG, Gonyea FB, Mikesky A (1986). "Exercise induced increases in muscle fiber number". Eur J Appl Physiol Occup Physiol. 55 (2): 137–41. doi:10.1007/BF00714995. PMID 3698999. S2CID 29191826.
- ^ Jansson Eastward, Kaijser L (July 1977). "Muscle adaptation to extreme endurance training in human". Acta Physiol. Scand. 100 (three): 315–24. doi:ten.1111/j.1748-1716.1977.tb05956.x. PMID 144412.
- ^ Gollnick PD, Armstrong RB, Saubert CW, Piehl M, Saltin B (September 1972). "Enzyme activeness and cobweb composition in skeletal musculus of untrained and trained men". J Appl Physiol. 33 (3): 312–ix. doi:10.1152/jappl.1972.33.iii.312. PMID 4403464.
- ^ Schantz P, Henriksson J, Jansson Due east (April 1983). "Accommodation of man skeletal musculus to endurance training of long duration". Clinical Physiology. 3 (ii): 141–51. doi:10.1111/j.1475-097x.1983.tb00685.10. PMID 6682735.
- ^ Monster AW, Chan H, O'Connor D (April 1978). "Activity patterns of human skeletal muscles: relation to muscle fiber type limerick". Science. 200 (4339): 314–vii. doi:10.1126/science.635587. PMID 635587.
- ^ Pattengale PK, Holloszy JO (September 1967). "Augmentation of skeletal muscle myoglobin by a program of treadmill running". Am. J. Physiol. 213 (3): 783–v. doi:x.1152/ajplegacy.1967.213.3.783. PMID 6036801.
- ^ Nielsen, OB; Paoli, F; Overgaard, K (2001). "Protective effects of lactic acid on strength production in rat skeletal musculus". Journal of Physiology. 536 (1): 161–166. doi:10.1111/j.1469-7793.2001.t01-i-00161.ten. PMC2278832. PMID 11579166.
- ^ Robergs, R; Ghiasvand, F; Parker, D (2004). "Biochemistry of practise-induced metabolic acidosis". Am J Physiol Regul Integr Comp Physiol. 287 (3): R502–516. doi:x.1152/ajpregu.00114.2004. PMID 15308499.
- ^ Wang, Y; Jardine, MJ (Nov 2011). "Benefits of exercise training in patients receiving haemodialysis: a systematic review and meta-assay". British Periodical of Sports Medicine. 45 (xiv): 1165–six. doi:x.1136/bjsports-2011-090558. PMID 21989854. S2CID 27583398.
- ^ "Overview of Movement Disorders - Encephalon, Spinal Cord, and Nervus Disorders". MSD Manual Consumer Version . Retrieved 24 June 2021.
- ^ Dumé, Belle (18 May 2007). "'Muscle racket' could reveal diseases' progression". NewScientist.com news service.
- ^ Poole, RM, ed. (1986). The Incredible Machine. Washington, DC: National Geographic Society. pp. 307–311. ISBN978-0-87044-621-iv.
- ^ Cruz-Jentoft, AJ; Sayer, AA (29 June 2019). "Sarcopenia". Lancet. 393 (10191): 2636–2646. doi:10.1016/S0140-6736(19)31138-9. PMID 31171417. S2CID 208792618.
- ^ Fuster, G; Busquets, S; Almendro, V; López-Soriano, FJ; Argilés, JM (2007). "Antiproteolytic furnishings of plasma from hibernating bears: a new arroyo for musculus wasting therapy?". Clin Nutr. 26 (5): 658–661. doi:ten.1016/j.clnu.2007.07.003. PMID 17904252.
- ^ Roy, RR; Baldwin, KM; Edgerton, VR (1996). "Response of the neuromuscular unit of measurement to spaceflight: What has been learned from the rat model". Exerc. Sport Sci. Rev. 24: 399–425. doi:10.1249/00003677-199600240-00015. PMID 8744257. S2CID 44574997.
- ^ "NASA Muscle Atrophy Research (MARES) Website". Archived from the original on 4 May 2010.
- ^ Lohuis, TD; Harlow, HJ; Brook, TD (2007). "Hibernating black bears (Ursus americanus) feel skeletal muscle poly peptide balance during winter anorexia". Comp. Biochem. Physiol. B. 147 (i): xx–28. doi:ten.1016/j.cbpb.2006.12.020. PMID 17307375.
- ^ Ebner N, Springer J, Kalantar-Zadeh K, Lainscak Thou, Doehner W, Anker SD, von Haehling S (July 2013). "Mechanism and novel therapeutic approaches to wasting in chronic disease". Maturitas. 75 (3): 199–206. doi:10.1016/j.maturitas.2013.03.014. PMID 23664695.
- ^ Chal J, Oginuma Thousand, Al Tanoury Z, Gobert B, Sumara O, Hick A, Bousson F, Zidouni Y, Mursch C, Moncuquet P, Tassy O, Vincent S, Miyanari A, Bera A, Garnier JM, Guevara G, Hestin K, Kennedy L, Hayashi Due south, Drayton B, Cherrier T, Gayraud-Morel B, Gussoni E, Relaix F, Tajbakhsh S, Pourquié O (August 2015). "Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy". Nature Biotechnology. 33 (ix): 962–9. doi:ten.1038/nbt.3297. PMID 26237517. S2CID 21241434.

- ^ Cè, Eastward; Rampichini, South; Limonta, Due east; Esposito, F (10 December 2013). "Fatigue effects on the electromechanical filibuster components during the relaxation phase after isometric contraction". Acta Physiologica. 211 (1): 82–96. doi:10.1111/apha.12212. PMID 24319999. S2CID 34744926.
- ^ Xu, Q; Quan, Y; Yang, L; He, J (January 2013). "An adaptive algorithm for the decision of the onset and outset of muscle wrinkle past EMG point processing". IEEE Transactions on Neural Systems and Rehabilitation Engineering. 21 (ane): 65–73. doi:10.1109/TNSRE.2012.2226916. PMID 23193462. S2CID 25169061.
- ^ Milder, DA; Sutherland, EJ; Gandevia, SC; McNulty, PA (2014). "Sustained maximal voluntary contraction produces independent changes in human being motor axons and the muscle they innervate". PLOS One. 9 (3): e91754. Bibcode:2014PLoSO...991754M. doi:x.1371/periodical.pone.0091754. PMC3951451. PMID 24622330.
Are Muscle Cells Controlled Individually Or In Groups,
Source: https://en.wikipedia.org/wiki/Skeletal_muscle
Posted by: foxpoldned1975.blogspot.com


0 Response to "Are Muscle Cells Controlled Individually Or In Groups"
Post a Comment